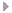| flash spectrum ...photography by Robert B Slobins |
General
Galleries
Coronal Spectrum, Kamilonga Farm - Zambia 21 June 2001

 Previous |
|
 Next |
With the sun at maximum, the Fe XIV line at 5303 Å shows as a ring. This is iron 13 times ionized, meaning that half of the electrons of these atoms are gone. These free electrons scatter sunlight to produce the continuous spectrum in the image. This charged gas in the inner corona is about 2 million degrees. This line's intensity relates to the level of solar activity. Another bright iron line, Fe X in the red at 6374 Å requires temperatures of only one million degrees.
Young and Harkness first observed the coronal spectrum in 1869. Analyses of spectra showed that this new green line could not be produced in the laboratory. In 1939, Grotrian and Edlen demonstrated that these so-called forbidden lines represented metals heated to extreme temperatures. However, the solar surface is only 5 800 degrees, so it appears that this contradicts the Second Law of Thermodynamics. Astrophysicists are still trying to solve this problem of coronal heating, although there is now evidence that magnetic Alfven waves propogating from the solar surface is part of this process.
Jupiter appears in the blue part of the spectrum.